ePartner Consulting Ltd
PO Box 1578
Lightwater
GU20 5AR
UK
Tel: 03300 100 000
Email: sales@epc.co.uk
Company registration number: 05192543
VAT number: GB842064740
Read our privacy policy.
ePartner Consulting Ltd
PO Box 1578
Lightwater
GU20 5AR
UK
Tel: 03300 100 000
Email: sales@epc.co.uk
Company registration number: 05192543
VAT number: GB842064740
Read our privacy policy.
- Home
- How it works
- Capture
Scanning paper documents and CRF’s
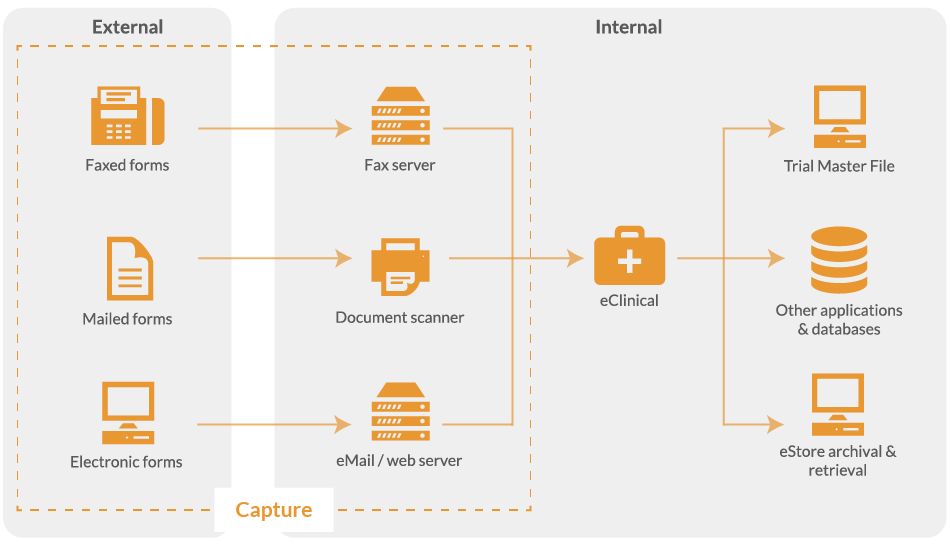
From scanning paper documents to completing eCRF’s in the browser, the data capture system adapts to the most appropriate method of data delivery.
Ease of capture for efficient processing
For paper-based clinical trials, all data collection forms are converted from a paper record to electronic image. This is achieved by scanning paper documents using a high-volume desktop scanner or direct from a fax line.
During medical document scanning, the image will undergo image pre-processing to check scan quality. This includes checking legibility, barcode readability, page size, presence of cornerstones etc.
Our document scanners start at 25 pages per minute and go up to over 100 pages per minute meaning the system will scan completed paper surveys in minutes – even data from large trials will be captured quickly. There is no need for manual sorting – the data capture system will do it for you, saving you even more time.
With eClinical, you do not need to send paper CRF’s to a scanning bureau as the data capture system enables on-site scanning of medical records, questionnaires and case report forms.
Document scanners

Panasonic KV-S1028Y
Max page size: A4
Pages per minute: 45
Feeder capacity: 100 sheets
Daily throughput: 100’s of sheets
RRP*: £450

Panasonic KV-S2087U
Max page size: A4
Pages per minute: 85
Feeder capacity: 200 sheets
Daily throughput: 1,000’s of sheets
RRP*: £1,100
* Prices subject to change and exclude VAT.
Electronic data capture (EDC) system
With the optional eForm module, eClinical can be used as a hybrid paper and electronic data capture (EDC) system.
Your paper form templates can be exported from the CRF designer as rich HTML or PDF eCRFs for participants and sites to intuitively fill-in on screen and submit using only a web browser.
Once complete, with your data rules enforced, the user may submit their eCRF electronically and/or print it for an ink signature. The printed CRF can later be posted or faxed back as a traditional paper form with the electronic data received immediately.
Seamless migration between paper and electronic case report forms
When electronic and/or paper forms are returned, they go through the same verification process before validated data is exported to your Trial Master File (TMF) or clinical trial management software (CTMS).
Whatever the data collection method, the data capture system captures and collates all CRFs from different sources, ready for processing.
If you want to learn more about scanning paper documents and CRF’s on-site with eClinical, or wish to book a demonstration, please contact us.
We also provide stand-alone EDC systems that simplify compliance with ICH GCP E6 R2 standards and other regulations such as 21CFR Part 11 with online forms, web interfaces, audit trails, access control, version tracking, electronic signatures and more.
Trusted by Rentokil Initial, Next plc, and the British Council, ePC support organisations to replace manual, paper-driven tasks with data capture, workflow, and document scanning solutions that reduce manual data entry and automate critical tasks. Visit our website.
Company registration number: 05192543. VAT number: GB842064740.
Address: PO Box 1578, Lightwater, GU20 5AR, United Kingdom.


