ePartner Consulting Ltd
PO Box 1578
Lightwater
GU20 5AR
UK
Tel: 03300 100 000
Email: sales@epc.co.uk
Company registration number: 05192543
VAT number: GB842064740
Read our privacy policy.
ePartner Consulting Ltd
PO Box 1578
Lightwater
GU20 5AR
UK
Tel: 03300 100 000
Email: sales@epc.co.uk
Company registration number: 05192543
VAT number: GB842064740
Read our privacy policy.
- Home
- How it works
- Export
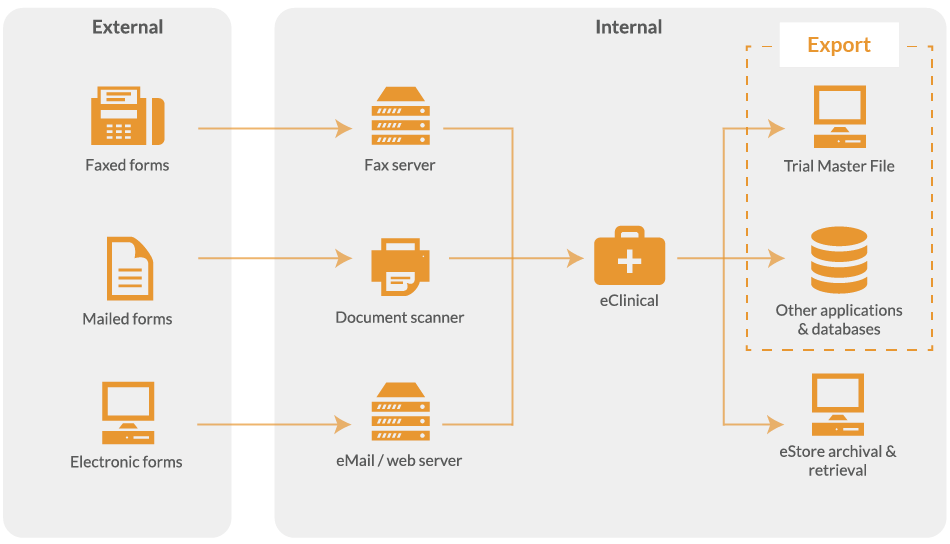
Export study data
Once verified, you are ready to export study data to common file formats (e.g. Excel, SPSS), Trial Master File (TMF) or Clinical Trial Management Software (CTMS) via the API.
Ease of export for seamless integration
The data capture system is compatible with many industry standard database systems for data validation lookups as well as exports.
The following formats are supported to facilitate ease of integration:
- Comma Separated Values (.CSV)
- XML (.XML)
- Microsoft access (.MDB or .ACCDB)
- Microsoft Excel (.XLS or .XLSX)
- SPSS (.SAV)
- ODBC data sources (for Microsoft SQL, Oracle, MYSQL etc.)
A copy of the digitalised paper CRF can be exported to a folder, document management solution, or our optional eStore storage and retrieval system for storing electronic records.
In addition to built-in exports, it is possible to create bespoke exports to web services or third-party systems via the API.
If you want to learn more about managing paper records in clinical trials, or wish to book a demonstration, please contact us.
Trusted by Rentokil Initial, Next plc, and the British Council, ePC support organisations to replace manual, paper-driven tasks with data capture, workflow, and document scanning solutions that reduce manual data entry and automate critical tasks. Visit our website.
Company registration number: 05192543. VAT number: GB842064740.
Address: PO Box 1578, Lightwater, GU20 5AR, United Kingdom.
